Hyperopic laser vision correction research study
Those interested in LASIK eye surgery may be eligible for a research study if they are farsighted and between 20 and 40 years of age.
The hyperopic laser vision correction clinical trial aims to correct farsightedness in a sufficient number of patients, enabling Bausch + Lomb to secure FDA approval for its advanced laser technology. Earlier this year, a study resulted in the FDA approving Bausch + Lomb’s laser technology for nearsightedness.
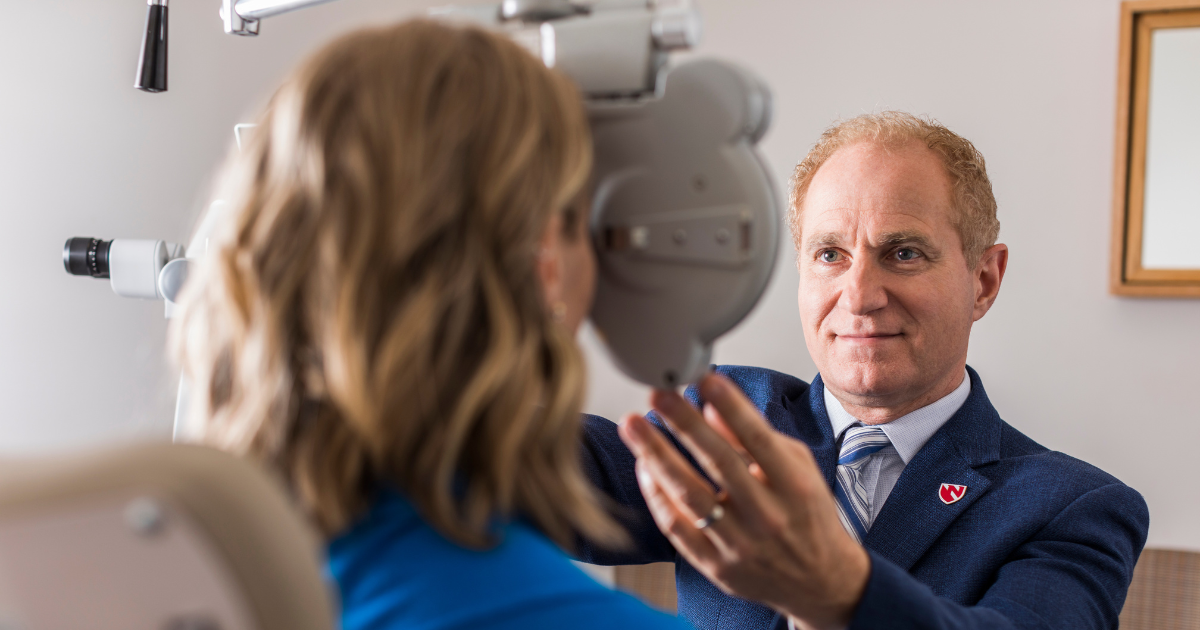
“About two years ago, we did a myopic, or nearsightedness, study of the laser, and now we are moving to the next stage, which is the hyperopic, or farsightedness, study,” explains ophthalmologist Ronald Krueger, MD, the trial’s principal investigator.
The study will enroll qualified patients and monitor them over the course of a year. To qualify for participation, individuals must be between 20 to 40 years old and diagnosed with farsightedness.
Trial participants will receive vision correction surgery, provided they commit to attending several follow-up visits throughout the year. “The ask that we have for patients is that, if they enroll, they will come back for all of the post-operative visits so we can gather the data,” Dr. Krueger says.
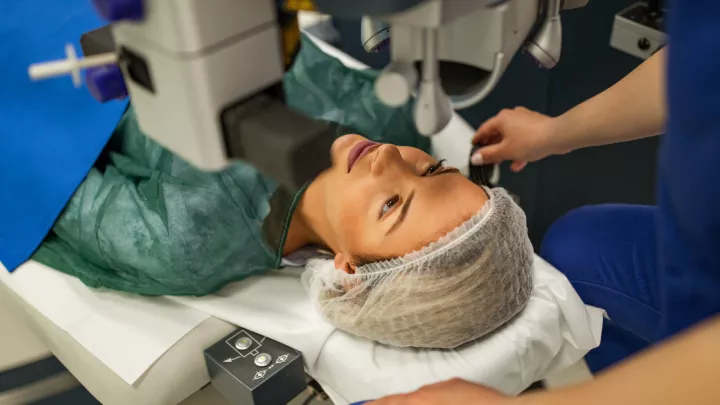
The procedure is a specialized form of LASIK surgery that involves using a specific type of laser to create a flap in the cornea – the eye’s outermost layer. Once the flap is lifted, the Bausch + Lomb study laser reshapes the cornea’s internal layers. After the reshaping, the flap is carefully repositioned, and the eye typically heals over time with a quick return of vision.
Potential participants must undergo a comprehensive screening exam to ensure they meet all the necessary criteria. This screening process, which takes approximately two to three hours, involves multiple tests to establish a baseline for the patient’s eye health and vision. The tests are crucial for comparing pre- and post-surgery visual outcomes to assess the procedure’s effectiveness.
If the trial proves successful, Bausch + Lomb could introduce a laser treatment for farsightedness to the U.S. market.
For more information, please contact Lisa Reid, ophthalmic clinical research supervisor, at 402.559.1851 or lisa.reid@unmc.edu.
IRB number 181-24-CB
Clinicaltrials.gov NCT05264623